Nickel Tetracarbonyl as Starting Material for the Synthesis of NHC‐stabilized Nickel(II) Allyl Complexes - Berthel - 2020 - Zeitschrift für anorganische und allgemeine Chemie - Wiley Online Library

Nickel Carbonyl, Preparation, Structure and Properties | Organometallic Chemistry | Inorganic Chem - YouTube
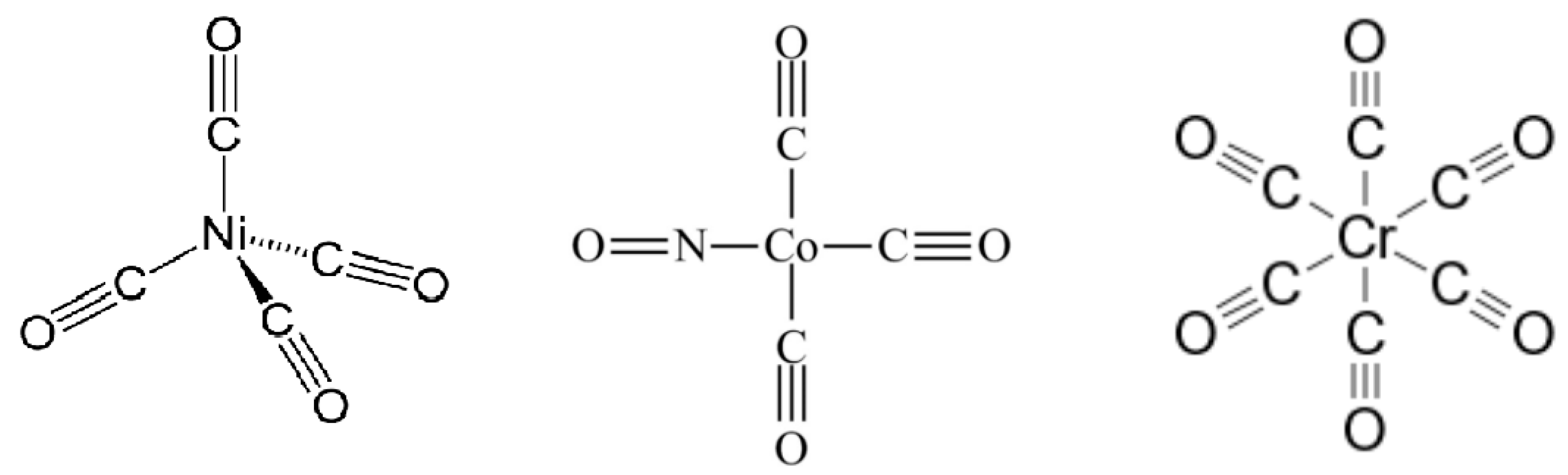
Chemistry | Free Full-Text | Dissociative Electron Attachment Cross Sections for Ni(CO)4, Co(CO)3NO, Cr(CO)6
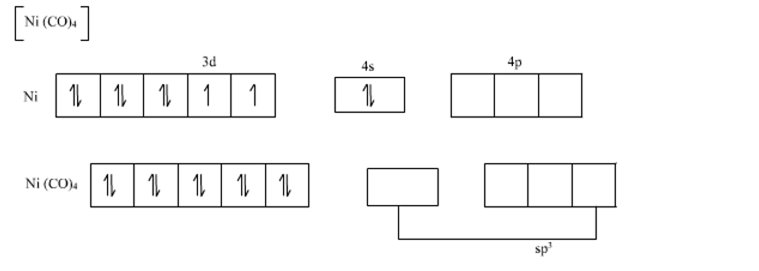
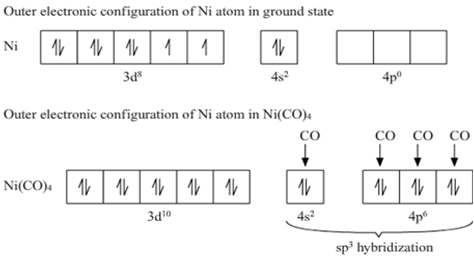
![Both [Ni(CO)4] and [Ni(CN)4]2− are diamagnetic. The hybridization of nickel in these complexes, respectively, are : Both [Ni(CO)4] and [Ni(CN)4]2− are diamagnetic. The hybridization of nickel in these complexes, respectively, are :](https://search-static.byjusweb.com/question-images/toppr_ext/questions/347646_32360_ans_241c88579f7a422aa5a21574b50a4e4e.png)




![Write the Hybridization and Magnetic Behaviour of the Complex [Ni(Co)4]. - Chemistry | Shaalaa.com Write the Hybridization and Magnetic Behaviour of the Complex [Ni(Co)4]. - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:0c935226f0c2451f9878137028e5360f.png)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-3.png)
![Ni(Co)4]explain hybridisation and structure of the above complex. - Brainly.in Ni(Co)4]explain hybridisation and structure of the above complex. - Brainly.in](https://hi-static.z-dn.net/files/db6/0b5b47f5c4df063603acf5d552d29fc2.jpg)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-2.png)
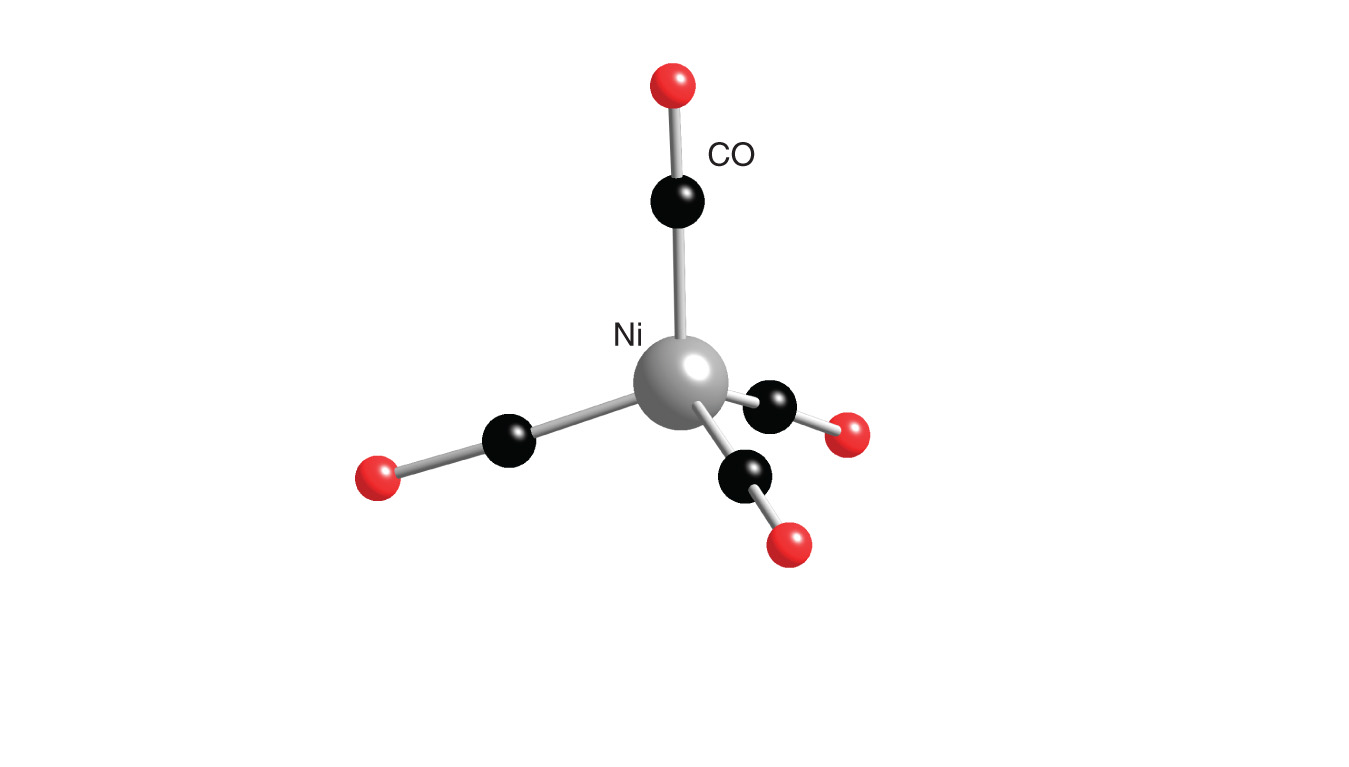

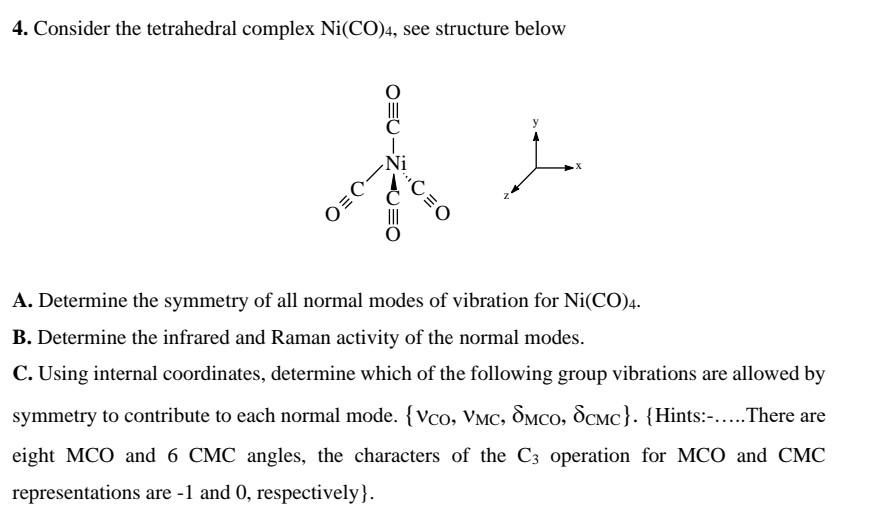

![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-1.png)
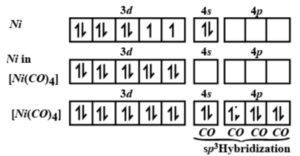
![Among [Ni(CO)4], [Ni(CN)4]^(2-), [NiCl4]^(2-) species, the hybridizati Among [Ni(CO)4], [Ni(CN)4]^(2-), [NiCl4]^(2-) species, the hybridizati](https://d10lpgp6xz60nq.cloudfront.net/physics_images/A2Z_CHM_XII_C09_E01_327_S01.png)