
NH4BF4 is added to a 2 M NH3 solution and heated. This is then added to hexaamminenickel(II) ion solution. What is the product? | Homework.Study.com
![Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube](https://i.ytimg.com/vi/R5RDFu1oYUU/maxresdefault.jpg)
Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube

The formation constant of `Ni(NH_3)_6^(2+)` is `6xx10^8` at `25^@C`.If 50 ml of 2.0 M `NH_3` is - YouTube
![C) 10 5. Which one of the following is an outer orbital complex and exhibits paramagnetin behaviour? (a) [Ni(NH3)6]2+ (b) [Zn(NH3)612+ (c) [Cr( NH3)6]3+ (d) (CO(NH3)6]3+ - T r action A + B C) 10 5. Which one of the following is an outer orbital complex and exhibits paramagnetin behaviour? (a) [Ni(NH3)6]2+ (b) [Zn(NH3)612+ (c) [Cr( NH3)6]3+ (d) (CO(NH3)6]3+ - T r action A + B](https://toppr-doubts-media.s3.amazonaws.com/images/8783536/3c02ac26-f23b-4dda-96f6-23fb3b82533e.jpg)
C) 10 5. Which one of the following is an outer orbital complex and exhibits paramagnetin behaviour? (a) [Ni(NH3)6]2+ (b) [Zn(NH3)612+ (c) [Cr( NH3)6]3+ (d) (CO(NH3)6]3+ - T r action A + B

Table 4 from Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate | Semantic Scholar
![Using VBT find the hybridisation of [Cr(NH3)6]2+ and [Ni(NH3)6]2+ - Chemistry - Coordination Compounds - 14079597 | Meritnation.com Using VBT find the hybridisation of [Cr(NH3)6]2+ and [Ni(NH3)6]2+ - Chemistry - Coordination Compounds - 14079597 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/content_ck_images/ck_3017c361558c5c239824ccfcefc3bac9.png)
2. The... | Download Scientific Diagram Representation of the cubic structure of [Ni(NH3)6](NO3)2. The... | Download Scientific Diagram](https://www.researchgate.net/publication/269400063/figure/fig8/AS:667921089036294@1536256205990/Representation-of-the-cubic-structure-of-NiNH36NO32-The-constituent-atoms.jpg)


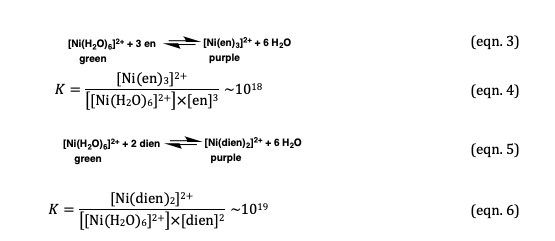
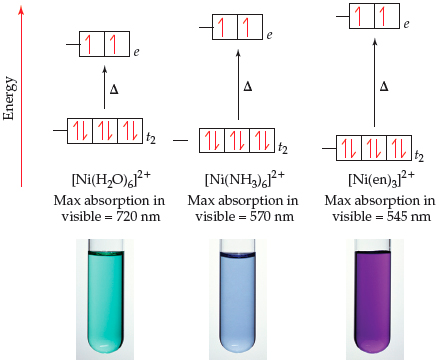

![Answered: [Ni (NH3) 6] Magnetic moment of 2+… | bartleby Answered: [Ni (NH3) 6] Magnetic moment of 2+… | bartleby](https://content.bartleby.com/qna-images/answer/c73807b6-45ed-4420-a3b1-cee7bec70749/203ec893-0265-480e-b058-95e1378e904d/3cda3a30-aca8-11eb-99b8-c16e6153b0d2_IMG_20210504_124206_523~2.jpg)

![Answered: [Ni (NH3) 6] C12 Explain the… | bartleby Answered: [Ni (NH3) 6] C12 Explain the… | bartleby](https://content.bartleby.com/qna-images/question/33dc3186-d7b9-4388-8560-215f799e8fe0/013f6e76-4df4-4063-bb5c-5eb17a580f72/z92qakc_thumbnail.png)
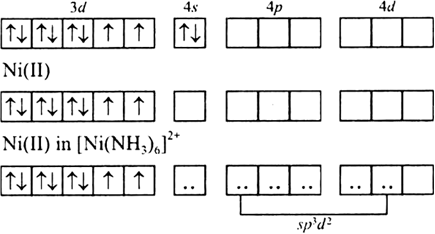

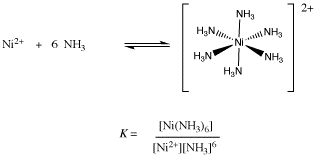
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-5256b41f5879418f6611bf7cb42000b7.webp)